TechTalk 1: Corrosion of Cylinders
We kick off our new TechTalk Series with the topic “Corrosion of Cylinders” and make you aware of our “Technical Literature” section here on our website.
Improper selection of material is one of the leading causes of failure in pressure vessels. It is important that you check the material suitability prior to order.
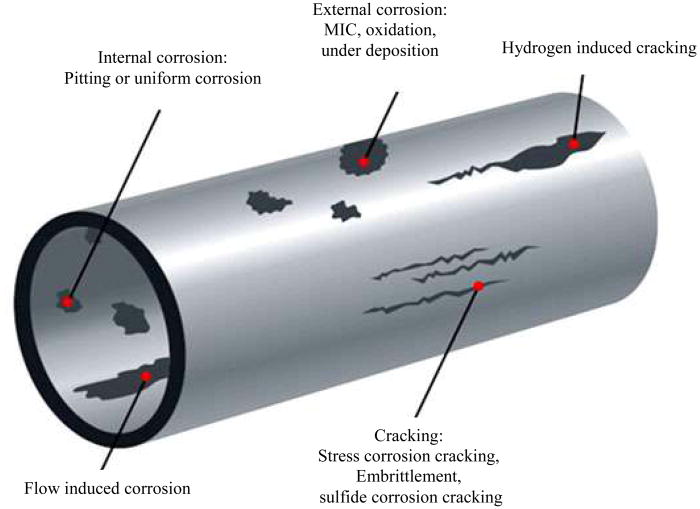
Types of Corrosion
Pitting is a localized form of corrosion in which cavities develop in the cylinder shell and by nature is much more difficult to detect. Pitting corrosion is most commonly found in stagnant vessels, as the water chemistry changes and causes a breakdown of the passive film. High concentrations of chloride can also cause pitting.
Stress Corrosion Cracking is caused by a combination of tensile stress and a corrosive environment. It is a very dangerous form of corrosion as most of the surface remains un-attacked but fine cracks can form causing disastrous failure to occur unexpectedly.
Galvanic Corrosion is a result of the coupling of dissimilar metals together in an electrolyte. When connected, a current will flow from one metal (the anode) to the other (the cathode). The anode will corrode, whilst the cathode will be protected.

Causes of Corrosion
Dissimilar Metals cause galvanic corrosion, therefore pipes, joints and fittings of dissimilar metals should not be connected within a system unless precautions are taken to prevent corrosion, such as the installation of dielectric separators.
Hard Water is high in dissolved mineral such as calcium and magnesium. When hard water is heated, lime scale can form. Hard water areas which generally have >280ppm have a high electrical conductivity and are therefore prone to galvanic corrosion.
Soft Water which has particularly low levels of CaCO3, often has a low pH-value and buffering capacity so can be very corrosive to metals.
High Chemical/Mineral/Bacterial Concentrations in the water supply can cause corrosive environments within the cylinder, which in combination with heat and pressure can cause Stress Corrosion Cracking.
Erosion Corrosion caused by turbulent flow due to excessive flow rates
Water Testing
Knowledge of the chemistry of the water supply at the site where the cylinder is to be installed is essential in order to determine whether or not the selected cylinder material will be suitable and whether water treatment should be considered. Factors that are important include; mineral content, dissolved gases (such as Oxygen, Carbon Dioxide and Hydrogen Sulfide), pH-value, hardness (in terms of CaCO3) and pH-level.
Manufacturing Processes
Welding of Stainless Steel is an aggressive process which, if not carried out correctly can greatly reduce the corrosion resistance of the material. World Heat uses a process called purging during the manufacture of stainless steel cylinders to limit oxidation. All of our domestic cylinders are then pickled internally, in order to allow the formation of the passive corrosion resistant layer. Copper cylinders are manufactured through the use of brazing or weld seaming using phosphorous wire. The inclusion of phosphorous means the base metal is not melted resulting in seams with a more consistent corrosion resistance.
Material Options
Contrary to popular belief all materials corrode, selection should be based on the rate at which it corrodes according to the vessels contents. When selecting a material it is also important to consider the following;
- Temperature and Pressure
- Water Properties (aggregate state, hardness, pH-value etc.)
- Further Conditions (start, shut-down etc.)
Stainless Steel grades 304 and 316 tanks may be at risk from Stress Corrosion Cracking (SCC) due to high Chloride levels in the water supply. An alternative to austenitic is Duplex Stainless Steel, its inherent low nickel levels make it more resistant to SCC.
Copper is less prone to rust and attack by chlorides, when problems do occur it is generally in low-use pipe work where stagnation can occur. High Ammonia/Manganese levels within the water supply will also increase the susceptibility of copper and brass to stress corrosion cracking and pitting.
Galvanized Steel cylinders are constructed from Carbon Steel to give the cylinder its strength and then hot-dip galvanized to prevent corrosion and make it suitable for use with potable water. All galvanized cylinders are supplied with a sacrificial anode as standard.
Anodes
Sacrificial Anodes use the principles of galvanic corrosion and ‘sacrifices’ itself for the protection of the cylinder. The corrosive material attacks the anode (the more easily corroded material), while the cylinder shell acts as the cathode. In soft water conditions, the low electrical conductivity of the water may reduce the electrical flow between the two metals, thereby stopping the anode from corroding and leaving the cylinder shell unprotected. In larger pressure vessels, sacrificial anodes are also not very efficient because of the large surface area of the cylinder.
Powered Anodes are therefore an option in both larger cylinders and soft water applications. Powered anodes are non-sacrificial, titanium anodes which, theoretically, should last the lifetime of the cylinder. They are connected to an external DC power source, which allows for a greater potential between the anode and cathode. In extremely soft water areas, powered anodes may also have no effect as the conductivity of the water may still be too low.
For domestic applications, World Heat strongly recommends powered anodes complete with indicator light to indicate the protection status. Sacrificial anodes require regular maintenance and will corrode at different rates depending on location.
Special Considerations for Commercial Vessels
Before large commercial vessels are used, it is important that they are thoroughly cleaned and flushed to remove any traces of manufacturing debris from both the vessel and system. Please contact a flushing specialist for further information. The vessel internals should be regularly inspected as part of a maintenance plan to check for any evidence of corrosion. Inspection openings are offered for this reason. During periods of downtime it is important that the water within the vessel is left to stagnate, thereby increasing the probability of corrosion. To avoid corrosion during these periods a number of options can be taken;
- Fill system with an inert gas (i.e. nitrogen)
- Fill and continuously circulate de-mineralized deoxygenated water
References
O’Brien et al (2014) A comparative performance analysis of Copper and Stainless Steel indirect hot water storage cylinders.
Reinhart, M (1975) Design for Corrosion Control of Potable Water Systems
www.pressurevesseltech.asmedigitalcollection.a sme.org
You can download our ‘FACT SHEET 1 – Corrosion of Cylinders’ directly from our website www.whcylinders.co.uk/technical-literature and check out any additional technical information you may require.



What are the common causes and conditions that lead to pitting corrosion in cylinders or vessels? How does the presence of stagnant water and high chloride concentrations contribute to the development of pitting corrosion?